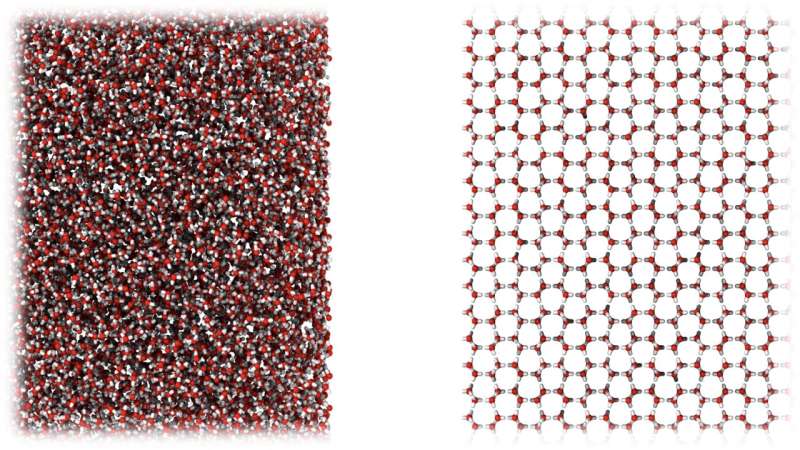
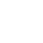Researchers from University College London (UCL) and the University of Cambridge, both in the United Kingdom, have discovered a new type of ice that more closely resembles liquid water than any other known ice and could rewrite our understanding of water and its many anomalies.
+ Discover the Futuristic and Luxurious Katara Towers of Qatar
+ Video shows Su-25 fighters destroying fortified positions in the Ukrainian War
+ Singer Doja Cat is generating rumors about the Illuminati after showing her new back tattoo
The newly discovered ice is amorphous, meaning its molecules are in a disordered form, unlike the organized structure found in common crystalline ice. Amorphous ice, although rare on Earth, is the primary type of ice found in space. This is because, in the colder environment of space, ice does not have enough thermal energy to form crystals.
New form
For the study, published in the journal Science, the research team used a process called ball-milling, vigorously shaking regular ice together with steel balls in a jar cooled to -200 degrees Celsius.
The researchers found that instead of ending up with small pieces of regular ice, the process produced a new amorphous form of ice that, unlike all other known ices, had the same density as liquid water and whose state resembled water in solid form. They called the new ice “medium-density amorphous ice” (MDA).
The team suggested that MDA (which looks like a fine white powder) could exist within ice moons of the outer Solar System, as tidal forces from gas giants like Jupiter and Saturn could exert shear forces (a deformation phenomenon in which the volume remains constant) similar to those created by ball milling on common ice. Furthermore, the team found that when MDA was heated and recrystallized, it released an extraordinary amount of heat, meaning it could trigger tectonic movements and “icequakes” in the kilometers-thick ice cover on moons like Ganymede (Jupiter’s largest satellite).